Soap Representation ActivityWith some pictures from a book by Brian Coppola at the end of the activity.
Materials needed for this activity:
Representations in this document: Click here for a summary of just the images. |
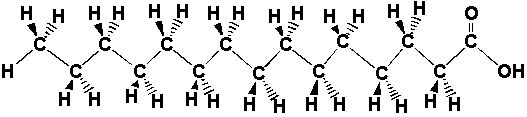
| The atoms in the fat molecule above are actually bonded together, but the three-dimensional picture doesn't show the bonds. We can show the bonds in two dimensions by drawing lines between the atoms. The two-dimensional picture below represents the same thing as the interactive picture above. Instead of colors, letters show which atoms are different. |
|
The bold bonds mean that the atom is
coming out of the page and the dashed
bonds mean that the atom is going back into the page. Look at this
two-dimensional picture and play with the three-dimensional picture
until you have convinced yourself that both
representations are equivalent. Sometimes all those hydrogens on the carbon atoms make the picture look busy or confusing, so we can just take them out. We know the hydrogens are there, but we leave them out of the drawing so that we can focus on the carbon and oxygen atoms, which form the "backbone" of the chain and determine how it reacts. Look at the three and two dimensional pictures below. They are just like the ones above except that the hydrogens that used to be on the carbon chain are gone, and we didn't put in all of the C's for carbon. |

| Notice that the molecule is starting to look like a snake! We could draw a more fanciful picture like this: |
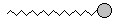
|
In this picture, the big gray bulbous head represents the oxygen-end of the
molecule, and the long squiggly tail is the chain of carbons and
hydrogens. This leaves out a lot of the details that we had in the
molecules above, but once again it stands for the same
thing!! In the representations above, the molecule looks like a fairly straight chain. The only reason for that is that it is easier to draw it that way!! In a real situation, the chain would be coiling and flopping all over the place like a worm. Take some gumdrops and thread them together on a string. Play with the chain for a while so you can feel what it would really be like. How would you represent this molecule? As a snake with a red tongue? As a tadpole with a bulbous head and a wriggly tail? As a bomb with a sparkly end? As a tower with water coming out of the top? Take a minute to think about this molecule and draw it. Be as imaginative as you can!! |

| Note that fats are non-polar. Although they have an oxygen on one end, most of their body is made up of just carbon and hydrogen atoms. |
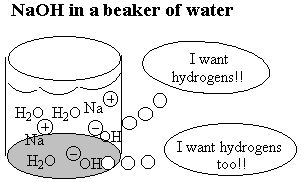
| The Fat Molecule | The Soap Molecule | 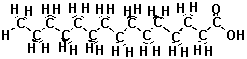 |
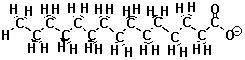 |
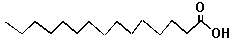 |
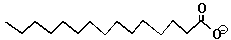 |
|---|
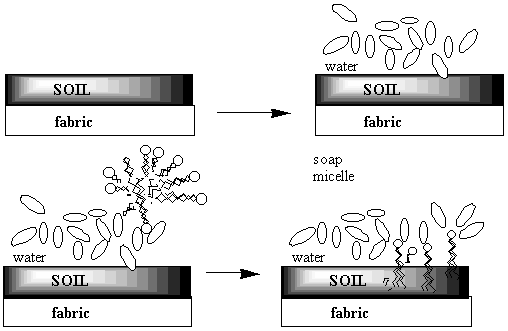
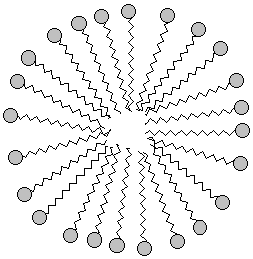
When the oxygen-end hydrogen is gone, the water becomes very attracted to the oxygen end of the fat molecule because removing the hydrogen makes the oxygen end of the molecule ionic and very polar. Now water, which is quite polar itself, just loves that end!! Unfortunately, the water doesn't like the long carbon chain that forms the rest of the molecule. Water shuns the carbon chains because if it has to be next to a carbon atom it can't be next to its fellow water molecules. Only the ionic oxygen ends are accepted into the water clique.Meanwhile, the non-polar, un-loved carbon and hydrogen atoms are pushed into a tight bubble so that the snobby water molecules never have to see them. Below is a three-dimensional picture of the cluster of fat molecules. In this picture, the hydrogen atoms are not shown, but remember that they are really there! Notice how the red oxygen ends of the chains are on the outside, where they can be in contact with the water molecules of the solution. The carbon and hydrogen chains, on the other hand, are never in contact with the water molecules floating around outside the bubble. Use your mouse to play with the picture so you can see it from all sides. Since it has many atoms, this image may be a little slower to rotate than the earlier ones--be patient!
|
|
If we wanted to show the cluster in two dimensions, we might draw it
like this:
|
|
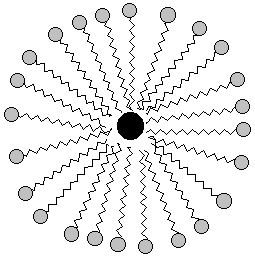
The pictures above are soap bubbles.
Soap bubbles form when base molecules steal hydrogen
atoms away from a fat molecules in water solution. The
reason soap cleans things is that water doesn't like dirt or grease
any more than it likes chains of carbon and hydrogen. So in a series
of steps the dirt and grease gets pushed into the inside of the soap
bubble. Then when you rinse away the soap bubbles, the dirt and
grease gets rinsed away too!!
Here's a two-dimensional picture of the soap bubble with some grease in the middle:
|
| How would you represent the molecules in a soap bubble?
Can you draw a picture of soap that is different from any of the ones
above? Be as creative as you can and feel free to use snakes or
tadpoles or magic wands or thought bubbles or anything that makes
sense to you. Then put some real soap in
water and look at the bubbles. Think about what's going on at the
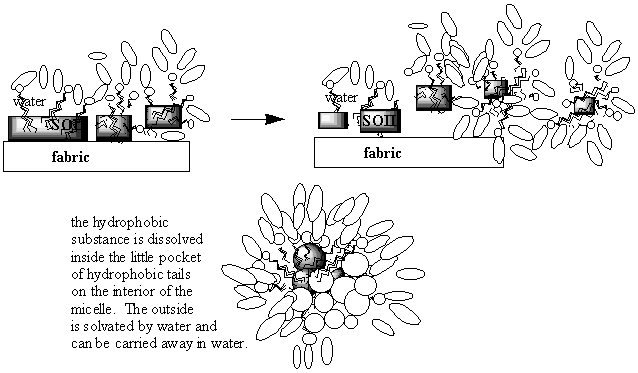
molecular level. Now that you are familiar with some of the different ways we can represent soap molecules, you are in a position to understand the series of steps in the cleaning process! Below are some pictures of what is going on. Look at them and think about what they are saying. Then see if you can explain in words what is happening to a friend. Lastly, write down an explanation of how soap molecules clean things in your ChemSense journal. Be sure to use lots of pictures!
|
| * The last series of images (above) were taken from Professor Brian Coppola's "Scrub-A-Dub" draft. |