|
SME Light Winter Quarter Lecture 11: What are some practical implications of chirality? Thursday, February 10th, 2000 Outline
I. Enantiomers and diastereomers Materials Needed:
I. Enantiomers and diastereomers We've learned that enantiomers are two molecules with opposite chiral centers. If there is only one chiral center in the molecule, then one enantiomer has that chiral center being R and the other enantiomer has that chiral center being S. But what if there are multiple chiral centers in the molecule? Then the situation is more complicated. Look at the picture of Vitamin E below.
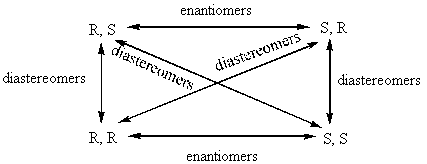
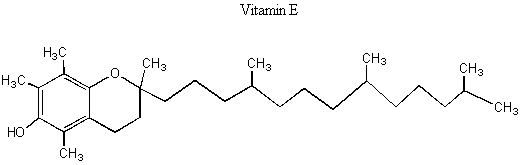 BREAK-OUT SESSION: How many chiral centers does Vitamin E have? How many different configurations could these chiral centers have in the molecule? (E.g. if there is one chiral center, it could be R or S. If there are two chiral centers, it could be RR, RS, SR, SS.) Vitamin E has three chiral centers, which means that it has eight possible configurations: RRR, SSS, RSR, SRS, RRS, SSR, RSS, SRR. The pairs that have opposite chirality at every chiral center (RRR and SSS, RSR and SRS, RRS and SSR, and RSS and SRR) are enantiomeric pairs because they are non-superimposable mirror images of each other. However, pairs of molecules with opposite chirality at only some centers are diastereomers. Diastereomers are stereoisomers with chiral centers that are not enantiomers. The following picture might help you visualize this.
 A. Meso Compounds The diagram above is unfailingly accurate in most cases, but there is one special case in which it breaks down. Consider R, S-1,2-dichloro-1,2-dibromoethane (don't worry about the nomenclature: just look at the picture):
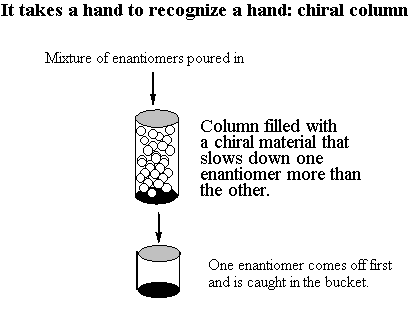
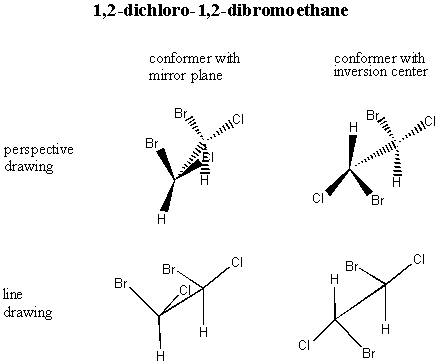 You would think that the R,S isomer and the S,R isomer would be enantiomers . . . but actually, they're the same compound! Build models of the R,S isomer and the S,R isomer to convince yourself of this. The internal mirror plane (or inversion center, depending on which conformer you choose to look at) renders the molecule achiral even though it has chiral centers. Such a compound is called a meso compound. You only have to watch out for meso compounds in cases where 1. The molecule is symmetric (same atoms on both ends) and 2. The chiral centers alternate configuration. For this molecule, the R,R and S,S versions are valid enantiomers. Build models so you can see this for yourself. B. The 2n Rule In general, if a molecule has n chiral centers, there are 2n different possible arrangements. For vitamin E, we had three chiral centers, so there were 23 = 8 possibilities. In the case where we had two chiral centers, there were 4 diastereomers. C. Chemical and Physical Properties There is an important difference between enantiomers and diastereomers. Enantiomers have all of the same physical properties as each other. They have the same boiling points, the same melting points, the same viscosity, etc. They can be dissolved in the same substances as each other. It is extremely difficult to separate a pair of enantiomers. There are two principle ways you can do it if you really want to. First, use what's called a "chiral column." A chiral column is filled with a chiral substance; if chosen properly, this substance will interact with the enantiomers as they pass through it and will slow them down differentially so that one particular handedness of enantiomer will come out first. You can put a container at the bottom to catch the pure enantiomer as it comes off, but you have to guess when to take the container away!!
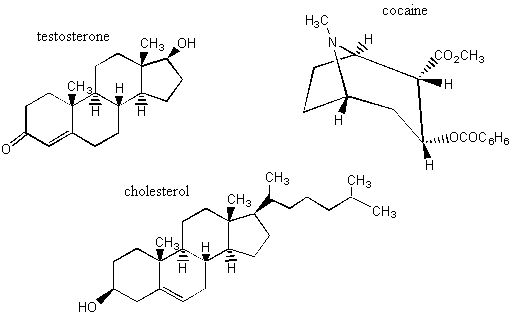
 The second way to separate enantiomers is to make them into diastereomers. This is the way that is most often used in industry to get enantiomers apart. If you had two enantiomers RSR and SRS and added an R chiral center, then you'd have RSRR and SRSR. RSR and SRS and enantiomers, but RSRR and SRSR are diastereomers. Why does making the enantiomers into diastereomers make it easier to separate them? Diastereomers have different physical properties. For example, the RSRR and SRSR components of the above mixture would have different melting points, boiling points, and solubilities. After being separated, the extra R chiral center is taken off again and the chemist is left with two separate buckets of RSR and SRS. No matter how you do it, though, it's never easy to separate enantiomers and diastereomers. This is made further relevant by the fact that when drugs or vitamins are made synthetically in a lab, all of the possible enantiomers and diastereomers show up in the mixture. It is difficult and expensive to separate them. BREAK-OUT SESSION: How would their different physical properties allow you to separate two diastereomers? Propose a separation technique for two diastereomers with different solubilities in water. In order to separate two diastereomers with different solubilities in water, all you'd have to do is dissolve the mixture in water and then filter out the undissolved diastereomer. In real life, you'd have to fiddle with the temperature to make sure that the other diastereomer really didn't dissolve and you probably wouldn't get a great enantiomeric excess unless the conditions were exactly right. But you get the idea. A. Here Piggy: Perfumes and the industry Most of the molecules that operate in our bodies are chiral, and moreover, only one of the many possible chiral forms is active! Look at some of the examples of chiral molecules that affect your body:
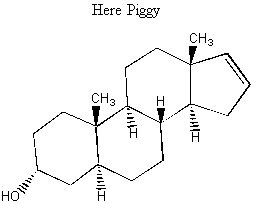
 Below is a molecule that is very attractive to female piggies. It is a component of truffles. This molecule is why female pigs are so good at rooting out truffles. Derivitatives of this molecule are commonly used in perfumes; several extremely sketchy-sounding studies have involved people coating chairs in a waiting room with various molecules like this and observing where people sit.
 B. Which Vitamin E would you buy? Fred and Tricia both take supplemental vitamin E. Fred buys his vitamin E from the local drugstore, but Tricia will only go to the health-food store. She pays a lot more, but believes that getting natural vitamin E, isolated from soybeans, is worth it. One day Fred and Tricia decide to see whether their vitamins are the same. They test the melting point of one of each of their pills. The melting points are different!! BREAK-OUT SESSION: Why is Tricia paying more? What is the difference between Tricia's and Fred's vitamin E? Which one would you buy? Tricia is paying more because she is getting a pure enantiomer. Nature only makes vitamin E in one way, and that way is the only way that is efficacious in your body. Other enantiomers and diastereomers won't work (and they may even be harmful). There are eight possible diastereomers of vitamin E, as we have seen. When the vitamin is made synthetically in the lab, all eight diastereomers are in the initial mixture, and it is difficult and expensive to separate them. So Fred is probably getting all eight. Given a choice, I'd go with Tricia's. The issue is further complicated by the fact that vitamins do not work in isolation in your body. Some studies have shown, for example, that people who get vitamins from food absorb them better than people who take synthetic or natural supplements. Some of this is readily explained by the solubilities of the vitamins. If you take vitamin E without any fat around to solvate it, it's not going to be absorbed by your body. But it's not always that simple. We do not know all the issues surrounding the extraction of vitamins. So if you want to be sure you are getting your vitamin E in a way that is efficacious in your body, don't take any pills--just eat the soybeans!
|