|
SME Light Winter Quarter Lecture 10: How Do We Know When A Molecule Is Chiral? Tuesday, February 8th, 2000 Outline
I. Chiral Centers Materials needed:
I. Chiral Centers For the molecules we have considered so are, it was relatively easy to examine the molecule as a whole to figure out whether it was chiral. But what if you have a huge gallumphing molecule like cholesterol, below?
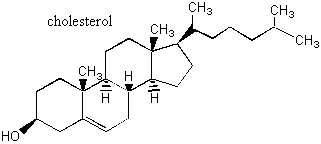 It would be very difficult to examine all possible conformations of this molecule to determine whether it was indeed chiral. One helpful shortcut is to look for chiral centers. Chiral centers are parts of the molecule where you see a carbon with four different things attached to it. A chiral center usually means that the molecule is chiral. This is not always the case, because a molecule might have two chiral centers but still be achiral because it is superimposable on its mirror image. (Such molecules usually have internal mirror planes or inversion centers. Molecules with internal mirror planes or inversion centers are always mathematically achiral. Mathematically, if an n-fold rotation followed by a reflection in a plane perpendicular to the rotation axis is a symmetry operation, then the molecule is achiral. Mirror reflection is 1-fold rotation followed by reflection in a plane perpendicular to the rotation axis and inversion symmetry is 2-fold rotation followed by reflection in a plane perpendicular to the rotation axis.) However, in the case of a big gallumphing molecule like cholesterol, you can see that this wouldn't be a concern. There's no fear of finding a mirror plane or an inversion center in cholesterol!! If a big molecule has one or more chiral centers, then it is a chiral molecule. How many chiral centers do the following molecules have?
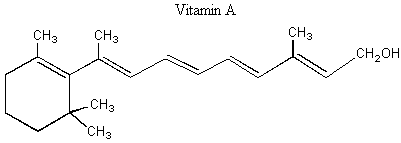
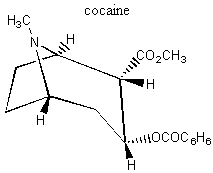
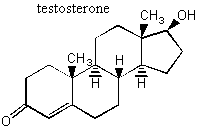
 II. Enantiomers Recall that enantiomers are stereoisomers that come about because of the tetrahedral geometry of the carbon atom. Enantiomers are chiral, or handed, and nature has designed biological systems such that they only recognize one handedness. If a molecule has more than one chiral center, then a pair of enantiomers of that molecule will have each and every chiral center with the opposite handedness. More on this at the end of the lecture. A. Spearmint and Caraway That only one chiral form of a given molecule is active in your body may seem a little hard to swallow at first, but it has vast and visible implications. Look at the following enantiomers:
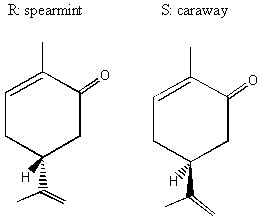 The only difference between the two enantiomers is the arrangement of the H on the bottom. Seems little . . . but in its "right-handed form," which we will learn to call R, this molecule is caraway. In its "left-handed form," which we will learn to call S, this molecule is spearmint! Changing only one little tiny geometrical arrangement of a carbon in this molecule makes it into a different thing entirely!! BREAK OUT SESSION: Why do the two different forms of carvone smell and taste different? Because receptors in our nose and our taste buds are themselves chiral and will react with the two different forms differently. It takes a hand to recognize a hand. B. The History of Thalidomide Note: Much of this history is quoted from chemist Roald Hoffman's famous book The Same and Not the Same.
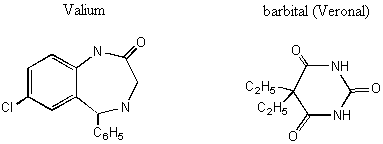
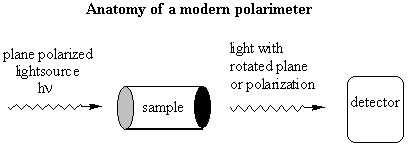
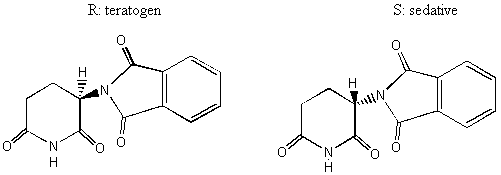 Notice the similarity between the chemical structures of thalidomide and the parts of the sedatives. Sedatives such as Valium were introduced as effective tranquilizers in the 1950's. There was a lot of money in the tranquilizer market, so companies began to experiment with compounds that were chemically similar, and one company in Germany ran across thalidomide. Nobody paid attention to the chiral center. They marketed it as a mixture of the 2 enantiomers. Hoffman writes: "Driven by the resemblance [between sedatives such as Valium and thalidomide], the Chemi Gruenenthal researchers convinced themselves that the molecule had good sedative properties. The reason I say it in this way is that subsequent investigations have failed to confirm the sedative qualities claimed. The toxicity of thalidomide was low, and this encouraged the manufacturers to put the drug on the market. It was first introduced as part of a drug combination directed toward respiratory infection in 1956 and shortly thereafter sold as a sedative directly and in dozens of combinations in Germany." The manufacturers needed published articles about thalidomide's effectiveness so they sought them out-but most of their "confirmations" were unfinished, unpublished, or verbal. Hoffman again: "In 1959 reports began to come in about severe neurological damage, neuritis, cased by thalidomide. These were steadfastly denied, obfuscated, and concealed by the Gruenenthal people; and numerous attempts were made to stifle public reporting of these symptoms. Only in Nov 1961 was the drug taken from the market." There is a famous picture in the Louvre of a woman showing another woman her thalidomide-deformed child. So, it turns out that thalidomide is a teratogen. But only in one of its isomers. With the other handedness, it is indeed the sedative that it was claimed to be. However, the matter is complicated by the fact that the "harmless" enantiomer converts into the "harmful" on under physiological conditions. The world is never simple . . . Other important pharmacological examples of chirality: D-penicillamine is a treatment for Wilson's disease, cystinuria, and rheumatoid arthritis; but enantiomer gives severe adverse side effects. The enantiomer of a tuberculosis drug, ethambutol, can cause blindness. Disastrous side effects associated with the painkiller benoxaprofn might have been avoided if the drug had been sold in its one-handed form. The 25 top-selling prescription drugs in the US sold for $34.4 billion in 1993. 25% were achiral, 11% were a mixture of two enantiomers, and 64% were one-handed. III. Enantiomers and plane polarized light A. Pasteur and his wines Chiral molecules were first identified when Louis Pasteur realized that they rotate plane polarized light. Louis Pasteur, a French chemist, was puzzled by the fact that crystals that formed on some wine bottles rotated plane polarized light in opposite directions. By coincidence, the crystals themselves were handed too, and Pasteur was able physically to separate the ones that rotated light clockwise from the ones that rotated it counterclockwise. Jean Baptiste Biot, the dean of French optical rotation studies, was skeptical and made Pasteur do it in front of him before he believed the result. It was a great moment in the history of chemistry. The fact that Pasteur could see which enantiomer was which just by looking at their crystals was a big coincidence. Normally you would not be able to tell just from looking which crystal was which. But the fact that, when dissolved in water, the differently handed crystals rotated plane polarized light was not at all a coincidence. In fact, the rotation of plane polarized light is the number one method of identifying enantiomers in modern chemistry labs. B. Polarimeters In order to identify the enantiomers, chemists make use of instruments called polarimeters. You will make a polarimeter in lab this week. The basic principle behind the operation of a polarimeter is summarized by the following picture:
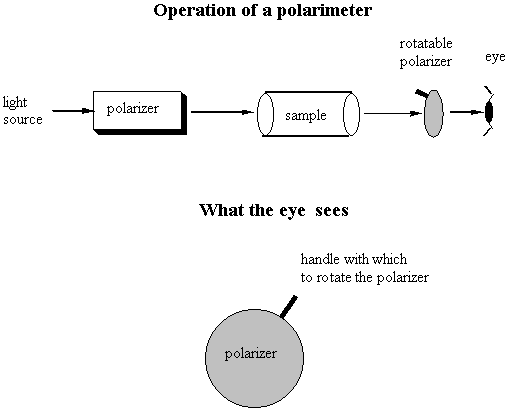 So, if you're looking through the eyepiece of the polarimeter and have the handle rotated so that polarization sheet is polarizing the light the same way that it was polarized going in to the sample chamber, the light would be slightly dimmer than it was going in. You would rotate the handle until you saw the brightest spot, and the number of degrees that you would have to rotate the handle would correspond to the number of degrees that the plane polarized light going in was rotated by its interaction with the sample. One thing that complicates this set-up is that different wavelengths of light are rotated different amounts. So you have to be careful about which wavelength is in your lightsource! Naming Chiral Centers Now that we know what chiral centers are, we have to devise a way of naming them. If you had the two molecules below and wanted to talk about the one on the left, how would you describe it to a friend who didn't have these same pictures in front of him?
 BREAK-OUT SESSION: Step 1: As a group: make a model of a single chiral center from gumdrops and toothpicks. Now make a model of the chiral center of opposite chirality. The two models that you have just made are called enantiomers. (When you get experienced at making these models you will realize that you need only change two of the atoms on the outside to switch between enantiomers.) Step 2: Have one group member choose one of the models but don't tell the rest of the group which one. Next slide: step #3: The group member who chose one of the models cannot consult the models again. Have that group member describe which enantiomer he or she chose on the index card. Then give the index card to the rest of the group. Could they figure out which one was intended? Pass up the index cards. BREAK-OUT SESSION: As a group, figure out how you would distinguish between enantiomers and write this down on the index card. Pass the index cards to the front of the class; we will put them on the web. How did you do it? Did you draw pictures? Or did you write something like "when the pink gumdrop is pointing out the red one is pointing back and up and the green one is on the left."? Both are good ways. Now let's see how chemists choose to name enantiomers. To name enantiomers Step 1: Prioritize the atoms The first thing to do is to "prioritize" the atoms from 1 to 4. The atom with the highest number on the periodic table gets priority number one. If two of the atoms are the same, you work along the chain until the first point of difference, and at that point the chain connected to the atom(s) with the highest atomic number(s) on the periodic table wins. If ever you hit a double bond, then that counts as being bonded to 2 of those kind of atoms. Step 2: Arrange the molecule so that the atom with lowest priority is pointing away from you Usually, the atom with lowest priority is hydrogen. In any case, make a picture or a model with the lowest priority atom pointing into the page. Then look at the other substituents. If they go clockwise from 1 to 3, the molecule is called "R." If they go counterclockwise, the molecules is called "S." Is the molecule you chose in the break-out session R or S? Try it with a friend for some other molecules. Do it with models and then also with pictures of more complicated molecules. Although this naming system is arbitrary, it is extremely powerful. Without it, we couldn't talk about enantiomers and expect other people to know which one we meant. Note if there is more than one chiral center in the molecule, enantiomers have to have each and every chiral center flipped in order to be enantiomers. IV. Enantiomeric Excess We know from previous lecture that chiral molecules rotate the plane of polarization of plane-polarized light. How much they rotate the light and in what direction is not something that can be predicted. Students always seem to make the mistake of thinking that S molecules rotate light counterclockwise and R molecules rotate light clockwise, but this is not the case. An S molecule might rotate light clockwise or counterclockwise, and it even depends on what wavelength of light you use! A molecule might rotate light clockwise in infrared wavelengths and counter-clockwise as the energy of the light gets higher. (As a standard, most rotations are measured from the sodium D-line, which is a specific wavelength.) For example, it is an experimental fact that at the sodium D-line, S-tartrate rotates the plane of polarization of light 11o clockwise and R-tartrate rotates the plane of polarization of light 11 o counterclockwise. A 50:50 mixture of R and S would not rotate the light at all, because the light that was rotated in one direction would get rotated right back the other way! The amount of rotation you can expect from many pure chiral compounds has been tabulated and checked by many experiments. Note that the amount that the light will be rotated depends on the length of the sample container, because the more chiral molecules the light has to pass through, the more it will be rotated. In a modern polarimeter, you just have to pop in your sample and get out a reading: you don't have to bother with turning handles and looking for maxima in the brightness of what you see.
 BREAK-OUT SESSION: Say you have a sample of limolene that rotates light 7o at the sodium D-line in a 10 cm cell. One pure enantiomer should rotate the light 10o. It hasn't been contaminated with anything that you can tell. What could this mean? What might have happened is that some amount of your pure enantiomer has changed into the enantiomer of the other conformation. This happens sometimes. Thalidomide, for example, is a harmless sedative in one chiral form, but a dangerous teratogen in the other handed from. The problem is that at biological conditions, the forms interconvert.
|